A potential new way to treat some of the most common blinding diseases
Inhibition of atypical protein kinase C may help manage macular edema and vision loss associated with eye diseases, reports The American Journal of Pathology
Many eye diseases, including diabetic retinopathy and macular degeneration, exhibit increased permeability of blood vessels in the macular (central) portion of the retina leading to abnormal fluid accumulation and vision loss. Therapies targeting a specific cytokine, vascular endothelial growth factor (VEGF), have transformed clinical care; however, not all patients respond well. A new report in The American Journal of Pathology shows that inhibiting a specific signaling molecule, atypical protein kinase C (aPKC), either genetically or pharmacologically, reduces increased vessel permeability and blocks inflammation. Blocking aPKC may help protect vision in patients with potentially blinding eye diseases.
“Our data reveal aPKC as an interesting target both for vascular permeability and inflammation and developing aPKC inhibitors may provide a new therapeutic option for blinding eye diseases,” explained lead investigator David A. Antonetti, PhD, Professor of Ophthalmology and Visual Sciences at The University of Michigan Kellogg Eye Center, Ann Arbor, MI, USA. “Our research may help patients with diabetic retinopathy, the leading cause of blindness in working age adults in the United States, and may also lead to new treatments for uveitis, a spectrum of diseases that leads to inflammation of the eye, as well as for retinal vein and artery occlusions.”
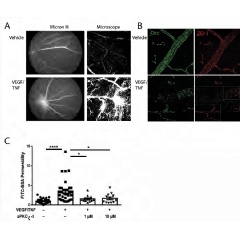
Good vision requires retinal neurons to send signals to the brain, and retinal neurons must be protected and kept in a healthy microenvironment within the eye. This microenvironment is maintained, in part, by the selectively permeable blood-retinal-barrier (BRB). The BRB includes the tight junctions between the endothelial cells of the blood vessels that help control entry of water, nutrients, and ions to the retina. However, injury or chronic disease can weaken the BRB and increase vascular permeability by altering these endothelial tight junctions. Studies have shown that a variety of molecular factors can affect permeability, including growth factor VEGF and the inflammatory cytokine tumor necrosis factor alpha (TNFα). Although VEGF and TNFα possess distinct signaling mechanisms, both eventually activate a common pathway, aPKC signaling, to change the permeability of the endothelial cells of blood vessels.
Further, aPKC promotes inflammation. In this study, investigators demonstrated the effects of VEGF and TNFα on retinal vascular permeability and the protective effect of an experimental small-molecule aPKC inhibitor using genetic mouse models and novel small molecule inhibitors to aPKC. The investigators also demonstrate the effect of targeting aPKC in a separate model of retinal inflammation. In both models, the genetic as well as therapeutic intervention reduced the vascular permeability and inflammation.
“This study evaluates the therapeutic potential of aPKC inhibition in retinal vascular permeability driven by inflammation and demonstrates that small molecule aPKC inhibitors have therapeutic potential for common ocular diseases,” commented Elizabeth A. Pearsall, PhD, Angiogenesis Laboratory of the Department of Ophthalmology at the Massachusetts Eye and Ear Hospital of Harvard Medical School, Boston, MA, USA. in an accompanying commentary. She noted that although there are still many unresolved questions about the etiology of inflammation, and whether it has a causative role in eye disease, additional pre-clinical studies necessary to bring small molecule aPKC inhibitors into clinical use are eagerly anticipated.
The recent advent of drug delivery to the eye provides an exciting opportunity to protect vision. The importance of good vision combined with the ability to deliver a drug in a focused and contained environment in the eye have led to the prospects of increasing therapeutic options to help individuals suffering from vision loss.
------
The article is “Inhibition of Atypical Protein Kinase C Reduces Inflammation-Induced Retinal Vascular Permeability,” by Cheng-mao Lin, Paul M. Titchenell, Jason M. Keil, Adolfo Garcia-Ocaña, Mark T. Bolinger, Steven F. Abcouwer, and David A. Antonetti (https://doi.org/10.1016/j.ajpath.2018.06.020). The commentary is “Atypical Protein Kinase C: Breaking Down Barriers in Ocular Disease?” by Elizabeth A. Pearsall and Kip M. Connor (https://doi.org/10.1016/j.ajpath.2018.07.006). Both will appear in The American Journal of Pathology, volume 188, Issue 10 (October 2018) published by Elsevier.
This study was funded in part by Supported by NIH grants R01 EY012021 (D.A.A.), R01 EY023725 (D.A.A.), and R24 EY024868 (D.A.A. and S.F.A.), Research to Prevent Blindness grants (D.A.A.), and JDRF grants (D.A.A.). This work used the Vision Research Core at the Kellogg Eye Center, funded by National Eye Institute grant P30 EY007003 and Morphology and Image Analysis Core of the Michigan Diabetes Research and Training Center grant P30 DK020572.
Full text of the study and editorial is available to credentialed journalists upon request; contact Eileen Leahy at +1 732 238 3628 or ajpmedia@elsevier.com. Journalists wishing to interview the authors should contact David A. Antonetti at dantonet@med.umich.edu. Elizabeth A. Pearsall may be reached for comment at Elizabeth_Moran@MEEI.HARVARD.EDU .
About The American Journal of Pathology
The American Journal of Pathology, official journal of the American Society for Investigative Pathology, published by Elsevier, seeks high-quality original research reports, reviews, and commentaries related to the molecular and cellular basis of disease. The editors will consider basic, translational, and clinical investigations that directly address mechanisms of pathogenesis or provide a foundation for future mechanistic inquiries. Examples of such foundational investigations include data mining, identification of biomarkers, molecular pathology, and discovery research. High priority is given to studies of human disease and relevant experimental models using molecular, cellular, and organismal approaches. ajp.amjpathol.org
About Elsevier
Elsevier is a global information analytics business that helps institutions and professionals advance healthcare, open science and improve performance for the benefit of humanity. Elsevier provides digital solutions and tools in the areas of strategic research management, R&D performance, clinical decision support and professional education, including ScienceDirect, Scopus, SciVal, ClinicalKey and Sherpath. Elsevier publishes over 2,500 digitized journals, including The Lancet and Cell, more than 38,000 e-book titles and many iconic reference works, including Gray’s Anatomy. Elsevier is part of RELX Group, a global provider of information and analytics for professionals and business customers across industries. www.elsevier.com
( Press Release Image: https://photos.webwire.com/prmedia/6/228765/228765-1.jpg )
WebWireID228765
This news content was configured by WebWire editorial staff. Linking is permitted.
News Release Distribution and Press Release Distribution Services Provided by WebWire.