Sensitive new assay detects hepatitis B infection in single liver cells and serum
Technique measuring the cccDNA marker may allow earlier detection of hepatocellular carcinoma in patients, reports The Journal of Molecular Diagnostics
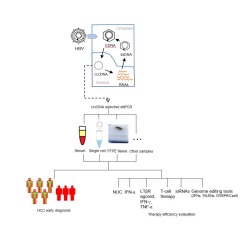
Chronic hepatitis B virus (HBV) can progress to cirrhosis and hepatocellular carcinoma (HCC). A studypublished in The Journal of Molecular Diagnostics describes a new HBV assay that offers advantages over currently used methods because it has the capability to detect closed circular DNA (cccDNA) in serum, single cells, and preserved tissue samples. This assay can be used to diagnose HCC at an earlier stage to manage treatment more effectively.
“The development of HCC is strongly associated with HBV. Recently, several new antiviral strategies targeting cccDNA have been established to improve HBV clearance. It is of great clinical significance to provide an accurate and sensitive approach for cccDNA detection. With this method, more and more patients with chronic HBV will have precision treatment available to prevent or delay HCC occurrence, and HCC in patients could be diagnosed at an earlier stage,” explained lead investigator Song-Mei Liu, MD, PhD, of the Center for Gene Diagnosis, Zhongnan Hospital of Wuhan University, Wuhan, China.
The new assay utilizes droplet digital PCR (ddPCR), which is highly sensitive and accurate for detecting trace molecules such as viruses. By combining ddPCR with PCR, the researchers were able to detect cccDNA in serum, single cells, and formalin-fixed, paraffin-embedded tumor issues. Southern blotting, currently considered the gold standard for this purpose, and other assays have important limitations like less sensitivity than ddPCR in detecting low copy numbers of cccDNA. Other methods require obtaining liver tissue through liver biopsy. “Compared to liver biopsy, serum can be obtained noninvasively, is widely used for clinical diagnostic purposes, and has a homogenous cccDNA distribution. The assay also improves the limit of detection of cccDNA,” noted Dr. Liu.
ddPCR was useful to identify which patients might be harboring hepatocellular carcinoma. Researchers found that almost 90 percent of 68 HCC patients were cccDNA-positive compared to 53 percent of 79 non-HCC patients. Serum cccDNA copy number was found to be higher in HCC patients compared to non-HCC patients. Combined analysis of serum cccDNA and HBV-DNA distinguished HCC patients from non-HCC patients. “This implicates cccDNA as a risk factor for HCC,” said Dr. Liu.
The investigators were also able to confirm that serum cccDNA was positively correlated with levels of cccDNA measured in liver samples. “Serum cccDNA is indeed a much better and useful diagnostic marker than intrahepatic cccDNA,” said Dr. Liu. Recently, several new antiviral strategies targeting cccDNA have been found to improve HBV clearance. Therefore, providing an accurate and sensitive approach for cccDNA detection is of great clinical significance for earlier diagnosis and HCC prediction, targeted treatment, and evaluating treatment efficacy.
In an accompanying commentary Fan Shen, MD, PhD, of the Department of Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada, observed, “Quantification of serum cccDNA and HBV-DNA is an effective way to discriminate HCC patients from non-HCC patients with a sensitivity of 74.5 percent and a specificity of 93.7 percent. This is an important finding and could lead to earlier diagnosis of HCC and more accurate prediction of chronic HBV progress in a noninvasive manner.”
According to the World Health Organization, 257 million individuals worldwide are infected with HBV, and in 2015 HBV resulted in 887,000 deaths, primarily due to liver complications. Chronic HBV infection can be treated with medications such as tenofovir or entecavir. In 2015, only nine percent of individuals living with HBV infection were aware they were infected.
------
The study is “A Highly Sensitive and Robust Method for Hepatitis B Virus Covalently Closed Circular DNA Detection in Single Cells and Serum,” by Jing-Tao Huang, Ying Yang, Yi-Min Hu, Xing-Hui Liu, Mei-Yan Liao, Roy Morgan, Er-Feng Yuan, Xia Li, and Song-Mei Liu ( https://doi.org/10.1016/j.jmoldx.2018.01.010 ). The editorial is “Commentary: HBV cccDNA—Selective Droplet Digital PCR: A Sensitive and Noninvasive Method for HCC Diagnosis?” by Fan Shen, Consolato Sergi, and Hui-lung Sun (https://doi.org/10.1016/j.jmoldx.2018.03.001). They will appear in The Journal of Molecular Diagnostics, volume 20, issue 3 (May 2018) published by Elsevier.
Full text of this study is available to credentialed journalists upon request; contact Eileen Leahy at +1 732 238 3628 or jmdmedia@elsevier.com. Journalists wishing to interview the study authors should contact Dr. Song-Mei Liu at +86 13971452926 or smliu@whu.edu.cn. Dr. Hui-lung Sun may be reached for comment at huilung@uchicago.edu.
The study was supported by grants from the National Natural Science Foundation of China (81772276, 81472023) and the National Basic Research Program of China (973 Program) (2012CB720600, 2012CB720605).
About The Journal of Molecular Diagnostics
[i]The Journal of Molecular Diagnostics[/i] , the official publication of the Association for Molecular Pathology, co-owned by the American Society for Investigative Pathology, and published by Elsevier, Inc., seeks to publish high quality original papers on scientific advances in the translation and validation of molecular discoveries in medicine into the clinical diagnostic setting, and the description and application of technological advances in the field of molecular diagnostic medicine. The editors welcome review articles that contain: novel discoveries or clinicopathologic correlations, including studies in oncology, infectious diseases, inherited diseases, predisposition to disease, or the description of polymorphisms linked to disease states or normal variations; the application of diagnostic methodologies in clinical trials; or the development of new or improved molecular methods for diagnosis or monitoring of disease or disease predisposition. http://jmd.amjpathol.org
About Elsevier
Elsevier is a global information analytics business that helps institutions and professionals advance healthcare, open science and improve performance for the benefit of humanity. Elsevier provides digital solutions and tools in the areas of strategic research management, R&D performance, clinical decision support and professional education, including ScienceDirect, Scopus, SciVal, ClinicalKey and Sherpath. Elsevier publishes over 2,500 digitized journals, including The Lancet and Cell, more than 38,000 e-book titles and many iconic reference works, including Gray’s Anatomy. Elsevier is part of RELX Group, a global provider of information and analytics for professionals and business customers across industries. www.elsevier.com
( Press Release Image: https://photos.webwire.com/prmedia/6/222697/222697-1.jpg )
WebWireID222697
This news content was configured by WebWire editorial staff. Linking is permitted.
News Release Distribution and Press Release Distribution Services Provided by WebWire.